Background
Reduced intensity conditioning (RIC) has expanded eligibility of older patients (pts) with hematological disorders for allogeneic hematopoietic cell transplant (HCT). Fludarabine and melphalan (FM) has been shown to improve disease control with an acceptable toxicity profile when combined with CNI-based GVHD prophylaxis regimens. The emergence of post-transplant cyclophosphamide (PTCy) as GVHD prophylaxis has improved outcomes of HCT across different HLA disparities and improved accessibility of HCT, especially in patient with no available matched donor. With recent data showing improved outcomes with RIC (Bolaños-Meade et al. NEJM 2023 and Shaw et al, JCO 2021), PTCy has been proposed as the standard of care for GVHD prophylaxis regardless of donor type. Herein, we report the largest experience with FM and PTCy as GVHD prophylaxis.
Methods
We retrospectively reviewed pts who underwent FM-based PBSC HCT with PTCy as GVHD prophylaxis at City of Hope from January 2015 to December 2021. Descriptive statistics were used to describe baseline characteristics. Kaplan-Meier Curves and log-rank tests were used to calculate and compare overall survival (OS) and disease-free survival (DFS), respectively. Cumulative incidence of relapse (CIR), non-relapse mortality (NRM) and GVHD were calculated and compared via a competing-risk analysis and Gray's test, respectively. Multivariate analyses (MVA) were performed using the multivariable Cox regression model for OS and DFS, and multivariable Fine and Gray regression model for the other variables. The primary aim was to evaluate the effect of donor types on HCT outcomes.
Results
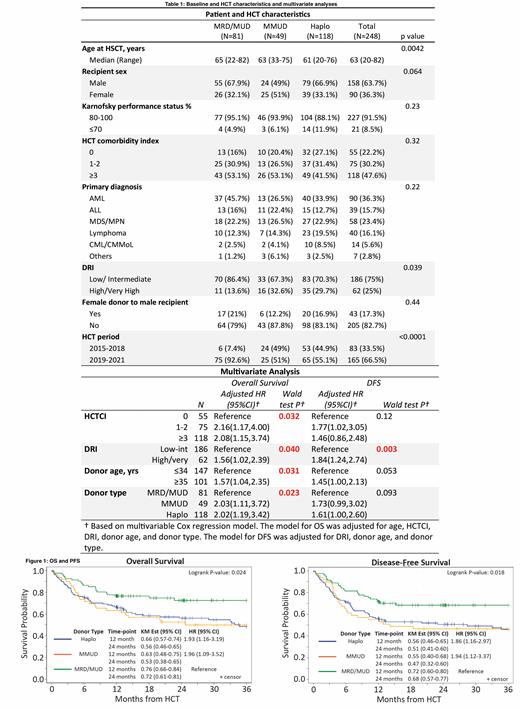
Baseline characteristics of 248 pts are summarized in Table 1. The median age was 63 (range, 20-82). 63.9% were male. The most common diagnoses were AML (n=90), MDS/MPN (n=58), lymphoma (n=40), and ALL (n=39). Of 248 pts, 89 (35.9%) received HCT from a matched related/unrelated donor (MRD/MUD), 118 (47.6%) from a haplo-identical (haplo) donor and 49 (19.8%) from a mismatched unrelated donor (MMUD).
The median times to neutrophil and platelet engraftment were 18 days (range, 18-19) and 32 days (range, 30-34), respectively. On MVA, MRD/MUD was associated with faster engraftment over haplo donors for neutrophils (hazard ratio [HR] 0.64, 95% CI, 0.47,0.87, P=0.015) and for platelets (0.49, 95% CI, 0.34-0.70, p<0.001), compared to MRD/MUD but there was no difference in engraftment between haplo and MMUD.
With a median follow-up for surviving pts of 24.4 months (range, 3.3-81.2), the 2-year OS and DFS for the all pts were 60.4% (95% CI, 53.7-66.5) and 55.5% (95% CI, 48.9-61.6), respectively. The 2-year OS and DFS for pts receiving haplo donors, MMUD, and MRD/MUD were 56.1%, 52.6%, and 72.5%, and 50.7%, 46.7%, and 68.4%, respectively (Figure 1). On MVA, compared with MRD/MUD, the 2-year OS was lower with haplo (HR 2.02, 95% CI, 1.19-3.42) and with MMUD (HR 2.03, 95% CI, 1.11-3.72 (p=0.023), while there was no difference between haplo and MMUD. Additionally, donor age >=35 years was associated with lower OS (HR 1.57, 95% CI, 1.04-2.35, p=0.031). There was a trend toward lower DFS with haplo (HR 1.61 (95% CI, 1.00-2.60)) and with MMUD, HR 1.73 (95% CI, 0.99-3.02, (p=0.093) when compared with MRD/MUD.
2-year NRM and CIR for all pts were 27.7% (95% CI, 22.1-33.5) and 16.8% (95% CI, 12.3-21.9), respectively. There were no differences in CIR based on donor type on MVA (p=0.87). NRM was higher in haplo and MMUD compared to MRD/MUD but this lost significance (p=0.13) in MVA after adjusting for KPS (HR 1.79, 95% CI, 0.98-3.26, p=0.019) and donor age >=35 years (HR: 1.87, 95% CI, 0.93-3.74, p=0.049). Day 100 CI of grade 2-4 and grade 3-4 acute GVHD for all pts were 39.5% (95% CI, 33.4-45.6) and 14.5% (95% CI, 10.5-19.2) and the 1-year CI of extensive chronic GVHD was 31.0% (95% CI, 25.2-37.0) There were no differences in grade 3-4 acute GVHD at day 100 or 1-year CI of extensive chronic GVHD based on donor type (p=0.46 and 0.13, respectively), although MVA revealed a strong trend towards a higher CI of grade 2-4 aGVHD at day 100 with MMUD group, HR 1.73 (95% CI, 1.02,2.95), p=0.079)
Conclusions
FM with PTCy was associated with promising disease control and acceptable NRM. Outcomes of pts undergoing MRD/MUD appears to be superior in this setting due to decreased NRM, while outcomes of pts undergoing HCT with haplo or MMUD are comparable. Donors <35 years was the strongest predictor of improved outcomes.
OffLabel Disclosure:
Koller:takeda: Consultancy, Speakers Bureau; treadwell therapuetics: Consultancy, Other: safety review committee; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Aldoss:Jazz: Consultancy; KiTE: Consultancy; Sobi: Consultancy; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; Takeda: Consultancy. Ali:BMS: Speakers Bureau; Blueprints: Speakers Bureau; Pharmaessentia: Consultancy; GSK: Consultancy; Karyopharm: Consultancy; Incyte: Research Funding. Salhotra:Rigel Pharma: Research Funding; Sanofi: Speakers Bureau; OrcaBio: Research Funding; Jazz Pharma: Research Funding; Kura Oncology: Research Funding; Gilead: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Aribi:Kite, a Gilead Company: Consultancy; Seagen: Consultancy. Artz:Abbvie: Consultancy; Astra Zeneca: Other: Advisory Board; Magenta Therapeutics: Other: Advisory Board; Radiology Partner: Current equity holder in private company, Other: Spouse equity interest. Becker:Accordant Health Services: Membership on an entity's Board of Directors or advisory committees; GPCR Therapeutics: Research Funding; Glycomimetics: Research Funding; Pfizer: Research Funding. Pullarkat:Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Stein:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Nakamura:BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Other: grant reviewer; Miyarisan: Research Funding; Sanofi: Consultancy; Napajen: Consultancy; Blue Bird: Consultancy; NCCN: Other: guideline panel for HCT; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; International Consortium: Other: consortium chair; Mt. Sinai: Other: Acute GVHD; Omeros: Consultancy. Al Malki:Tscan: Consultancy.
Cyclophosphamide is not approved for GVHD prevention

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal